Have you heard about the photoelectric effect? It's a fascinating phenomenon that played a key role in the history of physics. Let's dive into it with some animations!
Animation
Metal | Electron | Photon |
|---|---|---|
(Color depends on frequency) |
The photoelectric effect occurs when electrons are ejected from a metal surface when light (electromagnetic waves) shines on it. The electrons absorb energy from the light and are released.
Try adjusting the light intensity and frequency to see when electrons are emitted!
Key Features of the Photoelectric Effect
Since its discovery in the 19th century, scientists have uncovered several interesting properties of the photoelectric effect through experiments. Here are the main ones:
Key Features of the Photoelectric Effect
- Electrons are emitted instantly when light with a frequency above a certain threshold shines on the metal. Below this frequency, no electrons are emitted, no matter how intense the light is.
- Increasing the frequency of the light increases the maximum kinetic energy of the emitted electrons, but the number of electrons remains the same.
- For light above the threshold frequency, increasing the light intensity increases the number of emitted electrons, but their kinetic energy stays the same.
Is Light a Wave?
Back then, the prevailing belief was that light behaves like a wave. Many experiments supported this idea.
However, the characteristics of the photoelectric effect couldn't be explained if light were purely a wave.
For example, the fact that no electrons are emitted below a certain frequency, regardless of light intensity, was puzzling. If light were a wave, increasing its amplitude (intensity) should provide enough energy to eject electrons.
Einstein's Groundbreaking Idea
Albert Einstein, famous for his theory of relativity, tackled this mystery!
In 1905, Einstein proposed the quantum theory of light. This theory explained the photoelectric effect by treating light as particles called photons.
Photons have the following properties:
Photon Properties
A photon with frequency and wavelength has:
- Energy:
- Momentum:
Here,
Planck's constant and the speed of light are used.
Note that the basic wave equation is used.
In other words, photons have energy proportional to their frequency and momentum despite having no mass.
The idea that light behaves as both a wave and a particle might seem strange at first, but it beautifully explains the photoelectric effect. Let's break it down.
Explaining the Photoelectric Effect with Quantum Theory
First,
- Electrons are emitted instantly when light with a frequency above a certain threshold shines on the metal. Below this frequency, no electrons are emitted, no matter how intense the light is.
Why does this happen?
If we assume that each photon interacts with a single electron, this behavior makes sense.
Electrons are held in the metal by an attractive force. If a photon provides enough energy to overcome this force, the electron is released. Since photon energy is proportional to its frequency, electrons are emitted only when the frequency exceeds a certain value.
Specifically, the condition for electron emission is that the photon's energy exceeds the work function , which is the minimum energy needed to free the electron:
This is the condition for electron emission.
The work function is the minimum energy because the energy required for an electron to escape depends on its position in the metal. Electrons near the surface need less energy, while those deeper inside need more. The minimum energy is the work function.
The frequency at which electrons just start to escape is called the threshold frequency. The threshold frequency is given by:
Next,
- Increasing the light's frequency increases the maximum kinetic energy of the emitted electrons, but the number of electrons remains the same.
Why does this happen?
The kinetic energy of an electron is the photon's energy minus the energy needed to overcome the metal's attraction. So, the maximum kinetic energy is the photon's energy minus the work function.
Specifically, the maximum kinetic energy of an electron is given by:
Clearly, as the frequency increases, also increases.
The maximum speed of the emitted electrons is related by:
(Here, is the mass of the electron.)
The number of emitted electrons depends on the number of photons, not the frequency (as long as it exceeds the threshold frequency).
Finally,
- For light above the threshold frequency, increasing the light intensity increases the number of emitted electrons, but their kinetic energy stays the same.
Why does this happen?
This is straightforward. Increasing the light intensity means more photons, so more electrons are emitted. Remember, each photon interacts with a single electron.
The kinetic energy of the electrons is unaffected by the number of photons!
Everyday Examples of the Photoelectric Effect
Here are some real-world examples where the photoelectric effect can be observed:
Foil Electroscope
A foil electroscope is a device used to detect electric charge. For more details, check out this article:
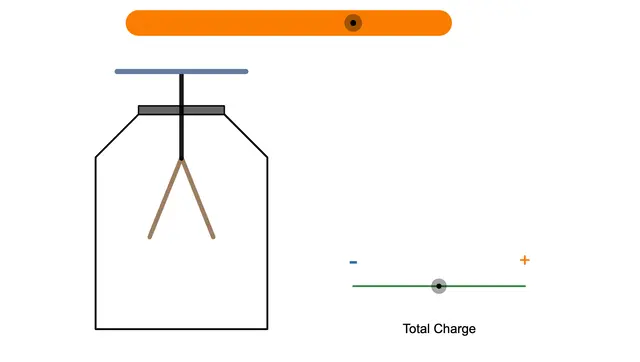
When ultraviolet or high-frequency light shines on the metal plate of a negatively charged foil electroscope, the foil closes.
This happens because electrons are emitted due to the photoelectric effect, reducing the negative charge.
If the electroscope is positively charged, the foil doesn't close. Can you figure out why?
Death Flash Phenomenon
The death flash phenomenon occurs when a strong camera flash shines on the circuit board of an electronic device, causing it to malfunction.
For example, a bug in the Raspberry Pi computer became famous because a camera flash could crash the entire system.
This happens due to the photoelectric effect. Electrons energized by the flash light create unintended currents in the circuit, causing the system to fail.
This phenomenon is also related to the photovoltaic effect, where light shining on a semiconductor generates an electromotive force.